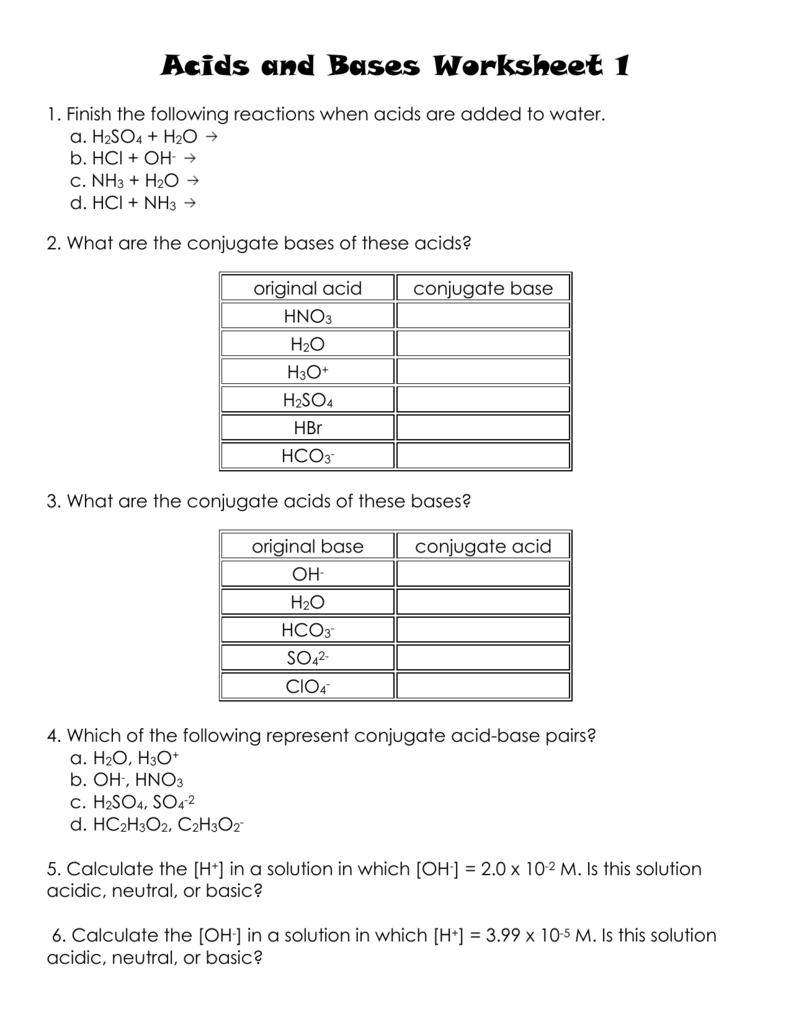
5 Essential Tips for Mastering Acids and Bases Calculations

Understanding acids and bases is fundamental in chemistry, influencing everything from industrial processes to everyday household tasks. Mastering calculations involving these substances is essential for any chemistry student or professional. Here are five crucial tips to help you excel in acids and bases calculations.
1. Understanding the Basics of pH and pOH

At the core of acids and bases calculations is the concept of pH and pOH. Here’s what you need to know:
- pH quantifies the acidity or basicity of a solution on a scale from 0 to 14. A pH of 7 is neutral, below 7 is acidic, and above 7 is alkaline.
- pOH similarly measures how alkaline a substance is. It’s calculated as 14 - pH for any solution at 25°C.
- Remember, pH + pOH = 14 at standard conditions.

2. Master the Concept of Molarity (M)

Molarity is key when dealing with solutions of acids or bases:
- It is defined as moles of solute per liter of solution.
- Formula for molarity: [ \text{M} = \frac{\text{moles of solute}}{\text{liter of solution}} ]
- When calculating concentrations, always ensure your units are in moles and liters.
🌡️ Note: Temperature affects volume; thus, molarity can change with temperature. Adjust calculations accordingly if the temperature isn’t standard.
3. Utilize the Equilibrium Constants

The strength of acids and bases is often determined by their dissociation constants (Ka for acids and Kb for bases):
- Ka (Acid dissociation constant) and Kb (Base dissociation constant) are measures of how well an acid or base dissociates in water.
- Key equations: [ K_a = \frac{[H^+][A^-]}{[HA]} ] [ K_b = \frac{[OH^-][HB^+]}{[B]} ]
- To find pH from these constants, use: [ pH = -\log([H^+]) ]
4. Apply the Henderson-Hasselbalch Equation

For buffer solutions or when titrating weak acids/bases, the Henderson-Hasselbalch equation is invaluable:
[ pH = pK_a + \log\left(\frac{[A^-]}{[HA]}\right) ]- This equation relates pH to the ratio of the conjugate base to the weak acid concentration.
- It’s particularly useful for understanding how to make or analyze buffer solutions.
5. Practice Titration and Equivalence Point Calculations
Titration is a technique for determining the concentration of an unknown solution:
- At the equivalence point, the moles of acid will equal the moles of base.
- Use the formula: [ M_1V_1 = M_2V_2 ] Where M is molarity, and V is volume.
- Titration curves can show you pH changes during the process, aiding in identifying the equivalence point.
By consistently applying these tips, your proficiency in handling acids and bases calculations will significantly improve. Remember to practice regularly, as each calculation reinforces your understanding of these fundamental chemical principles.
In sum, by mastering the concepts of pH, pOH, molarity, equilibrium constants, the Henderson-Hasselbalch equation, and titration calculations, you'll be well-equipped to handle almost any acid-base problem thrown your way. Each concept builds upon the others, providing a comprehensive framework for understanding the intricate dance of ions in solution.
How can I tell if a solution is acidic, neutral, or alkaline?

+
Use pH paper or a pH meter. Solutions with pH below 7 are acidic, pH of 7 is neutral, and above 7 is alkaline.
What are the practical applications of knowing acid and base concentrations?

+
They are crucial in medicine, environmental science, water treatment, and industries like food processing, pharmaceuticals, and agriculture.
How do I prepare for an acid-base titration exam?

+
Practice titration calculations, understand buffer solutions, and know how to interpret titration curves. Regularly test your skills with past exams and focus on mastering the key concepts like pH calculations and Henderson-Hasselbalch equation.