7 Proven Ways to Ace Your Isotopes Worksheet

Mastering isotopes and their properties is a fundamental aspect of any chemistry curriculum. Whether you're a student working through your first year of chemistry or someone looking to brush up on their scientific knowledge, understanding isotopes can significantly enhance your grasp of atomic theory and its applications. Here, we explore seven proven strategies to help you ace your isotopes worksheet with confidence and comprehension.
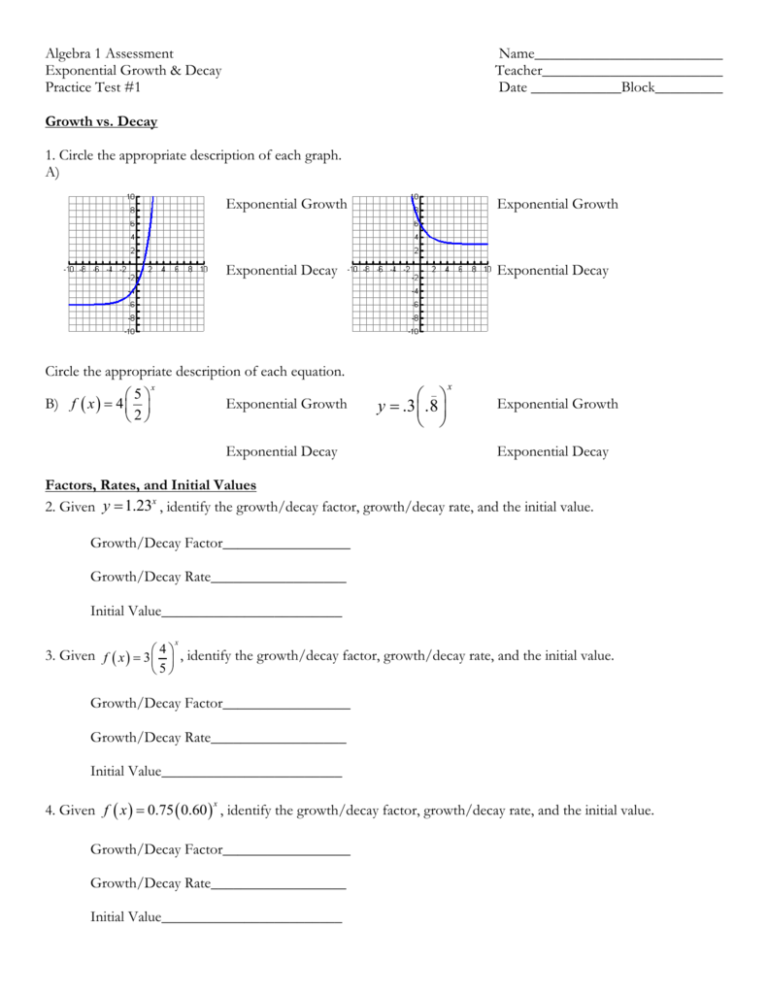
1. Understand the Concept of Isotopes

Begin by ensuring you have a clear understanding of what isotopes are:
- Isotopes are variants of a particular chemical element that differ in neutron number, and consequently in their mass number.
- They have the same number of protons, hence the same atomic number, but different numbers of neutrons.
An example can make this clearer:
| Element | Isotope | Protons | Neutrons |
|---|---|---|---|
| Carbon | C-12 | 6 | 6 |
| Carbon | C-14 | 6 | 8 |

2. Master Isotopic Notation

Isotopic notation is crucial for accurately describing isotopes. Here’s how you can write isotopes:
- AXZ, where A is the mass number (protons + neutrons), X is the element symbol, and Z is the atomic number (protons).
- For instance, carbon-14 is written as 14C6.
3. Utilize Visual Aids
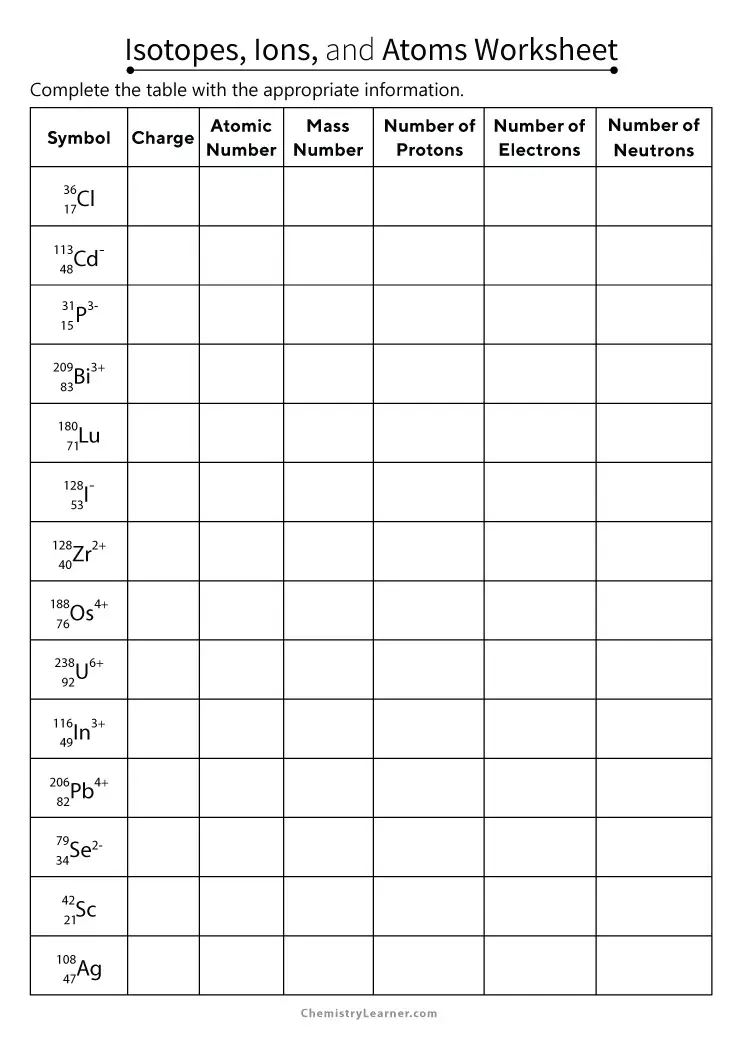

Visual learning can significantly aid in memorizing isotopic differences:
- Draw diagrams illustrating isotopic variations for key elements.
- Use colors to differentiate protons from neutrons in nucleus representations.
🔎 Note: Ensuring visual aids are neat and clear helps in grasping complex concepts quickly.
4. Practice Calculating Atomic Mass

The atomic mass on the periodic table reflects the average mass of all the isotopes of an element, taking into account their natural abundance. Here’s the formula:
- Atomic Mass = (Mass of isotope 1 × abundance) + (Mass of isotope 2 × abundance) + …
5. Memorize Key Isotopes and Their Uses

Knowing common isotopes and their applications can give you an edge:
- Carbon-14 (C-14) for dating archaeological samples.
- Uranium-235 (U-235) for nuclear reactors and atomic bombs.
6. Apply Real-World Examples

Relating isotopes to real-world scenarios can make learning more engaging:
- Discuss how isotopes like Cobalt-60 are used in cancer treatment.
- Explore how Hydrogen isotopes affect water chemistry.
7. Use Practice Worksheets and Quizzes

The best way to solidify your understanding is through practice:
- Seek out or create practice worksheets that focus on isotopic calculations and concepts.
- Use online quizzes or interactive resources for quick feedback and reinforcement.
By following these steps, your journey through the world of isotopes will not only be educational but also fascinating. Isotopes are not just abstract numbers; they have practical applications in medicine, archaeology, energy production, and beyond. With a comprehensive understanding of isotopes, you'll be able to tackle any worksheet with confidence, ensuring you're well-prepared for your next quiz or exam. Remember, mastery in any subject comes from consistent practice, deep understanding, and a willingness to explore beyond the basics.
What are the differences between isotopes?

+
Isotopes of an element differ in their number of neutrons. This variation leads to differences in mass and potentially in stability and nuclear properties, but not in chemical behavior as they have the same number of protons and electrons.
Why do we need to know about isotopes?

+
Isotopes have significant applications in fields like chemistry, physics, geology, medicine, and even archaeology, affecting how we understand atomic structures, date historical artifacts, treat diseases, and analyze environmental samples.
How can isotopes be used in dating materials?

+
Some isotopes, like Carbon-14, are radioactive and decay over time. By measuring the ratio of the radioactive isotope to its stable counterpart in a sample, we can determine the age of organic material since the rate of decay is constant and known.