5 Key Facts on Atom's History: Worksheet Guide

Atoms, the fundamental building blocks of matter, have a rich history filled with groundbreaking discoveries and evolving theories. This worksheet guide will delve into five key facts about the history of atomic theory, providing an insightful journey from ancient thoughts to modern quantum leaps. Whether you're a student, teacher, or an enthusiast, understanding the atom's history is crucial for grasping the complexities of chemistry and physics.
1. Ancient Theories on the Atom

The concept of atoms wasn't born in the modern era but dates back to antiquity. Here are the key points:
- Leucippus and Democritus: Around 440 BC, these Greek philosophers introduced the idea of 'atomos' or indivisible particles. They suggested that the world was made up of empty space and solid, indivisible parts.
- Ancient Indian Philosophy: Concepts similar to atoms existed in Hindu thought, notably in the work of Kanada from the Vaisheshika school, who hypothesized that everything is made of "Anu" or small indestructible particles.
- The understanding of atoms was purely philosophical, lacking empirical evidence or experimental backing.
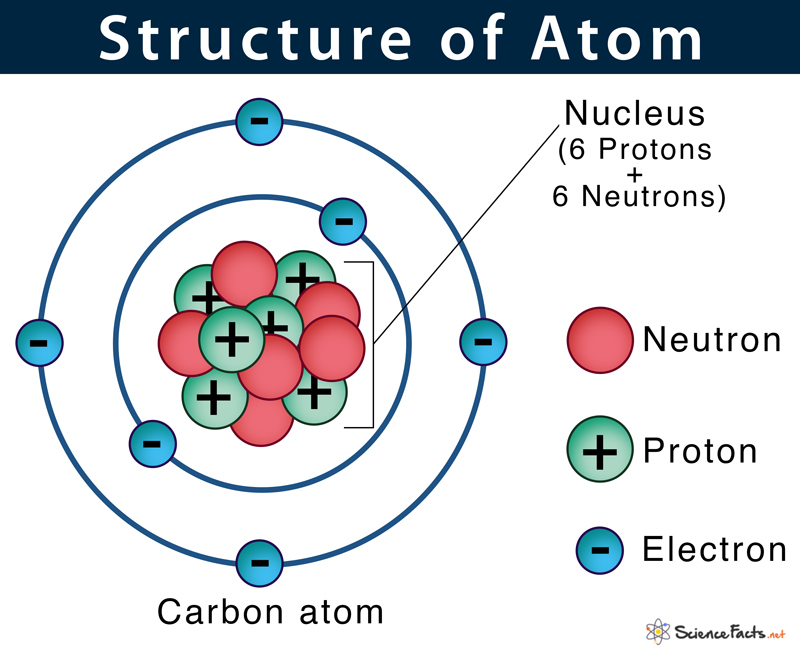
2. John Dalton's Atomic Theory

In the early 19th century, John Dalton presented a more scientific approach to atomic theory:
- He proposed that elements consist of atoms, which are indestructible and identical for a given element.
- Compounds form when atoms combine in simple, whole-number ratios.
- His model could explain laws of chemical combination, like the law of definite proportions.
- However, Dalton's theory did not account for isotopes or the complex nature of atomic structure.
| Dalton's Contribution | Current Understanding |
|---|---|
| All matter is made of atoms | True, but atoms are divisible into protons, neutrons, and electrons. |
| Atoms are indivisible and indestructible | Partially true; atoms can be split into subatomic particles. |
| Atoms of the same element are identical | Not entirely correct due to isotopes. |
| Chemical reactions involve the rearrangement of atoms | Still true and foundational to chemical reactions. |

📢 Note: Dalton’s atomic theory marked a pivotal shift towards empirical evidence and provided a framework for future chemical discoveries.
3. J.J. Thomson's Plum Pudding Model

By the late 19th century, discoveries in electricity and subatomic particles led to a model:
- Thomson discovered the electron, challenging Dalton's view of the atom as indivisible.
- His "Plum Pudding Model" envisioned atoms as a positively charged substance with embedded negatively charged electrons.
- This model, while flawed, was an essential stepping stone in understanding atomic structure.
4. The Nuclear Atom: Rutherford's Experiments

Ernest Rutherford's gold foil experiment in 1911 reshaped atomic theory:
- He bombarded gold foil with alpha particles and observed unexpected deflections, leading to the discovery of the atomic nucleus.
- His nuclear model proposed that an atom has a dense central nucleus containing most of its mass and positive charge, with electrons orbiting at a distance.
- These findings laid the groundwork for understanding radioactivity, atomic energy, and nuclear fission.
5. The Quantum Leap: Bohr’s Atomic Model

Niels Bohr's model of the atom, introduced in 1913, integrated quantum theory:
- He proposed discrete energy levels for electrons, solving issues with the classical model's failure to explain the stability of atoms.
- Electrons can jump between energy levels by absorbing or emitting photons of specific energies.
- Bohr’s model could explain phenomena like the atomic spectra and the Balmer series for hydrogen.
🔬 Note: These findings and models from the early 20th century provided the foundation for modern quantum mechanics and the subsequent discovery of the true complexity of atomic structure.
From philosophical musings to precise scientific models, the history of atomic theory showcases humanity's quest to understand the very fabric of the universe. These developments not only provide a timeline of scientific progress but also highlight the dynamic interplay between theory and experiment. The atom, once considered the smallest unit of matter, has revealed layers of complexity, from the presence of subatomic particles to the strange world of quantum mechanics. This journey reflects not just the evolution of science but also the human spirit's relentless pursuit of knowledge. Each theory, model, and discovery has built upon the last, creating a robust and continuously expanding understanding of the atom, laying the groundwork for further advancements in physics, chemistry, and beyond.
Why were ancient theories on atoms purely philosophical?
+
Ancient thinkers lacked the experimental tools and scientific methods we have today, relying instead on logical reasoning and observation to conceptualize the world’s composition.
What was the major flaw in Dalton’s atomic theory?

+
Dalton’s theory did not account for isotopic variations within elements or the existence of subatomic particles, which later research revealed.
How did Thomson’s discovery of the electron change atomic theory?

+
His discovery provided empirical evidence that atoms were divisible, challenging the idea of atoms as the smallest, indivisible units of matter.
What significance did Rutherford’s experiments have on atomic theory?

+
They led to the realization that atoms are mostly empty space, with a dense nucleus at the center, fundamentally reshaping our understanding of atomic structure.