2.4 Chemical Reactions and Enzymes Worksheet Answers Revealed

Introduction to Chemical Reactions and Enzymes

Chemical reactions are the cornerstone of life, transforming simple elements and compounds into more complex structures, driving everything from cellular processes to the diverse metabolic pathways within organisms. In these intricate dance steps of molecules, enzymes play a pivotal role. Enzymes, known as biological catalysts, are crucial for speeding up these reactions, often under conditions that are far from what traditional chemistry would require. This blog post aims to delve into the fascinating world of chemical reactions and enzymes, offering insights into how these processes work, how enzymes catalyze reactions, and how their unique properties can be understood through various worksheets and study materials. Let's explore how these tiny molecular machines work to fuel life's processes.
Understanding Chemical Reactions
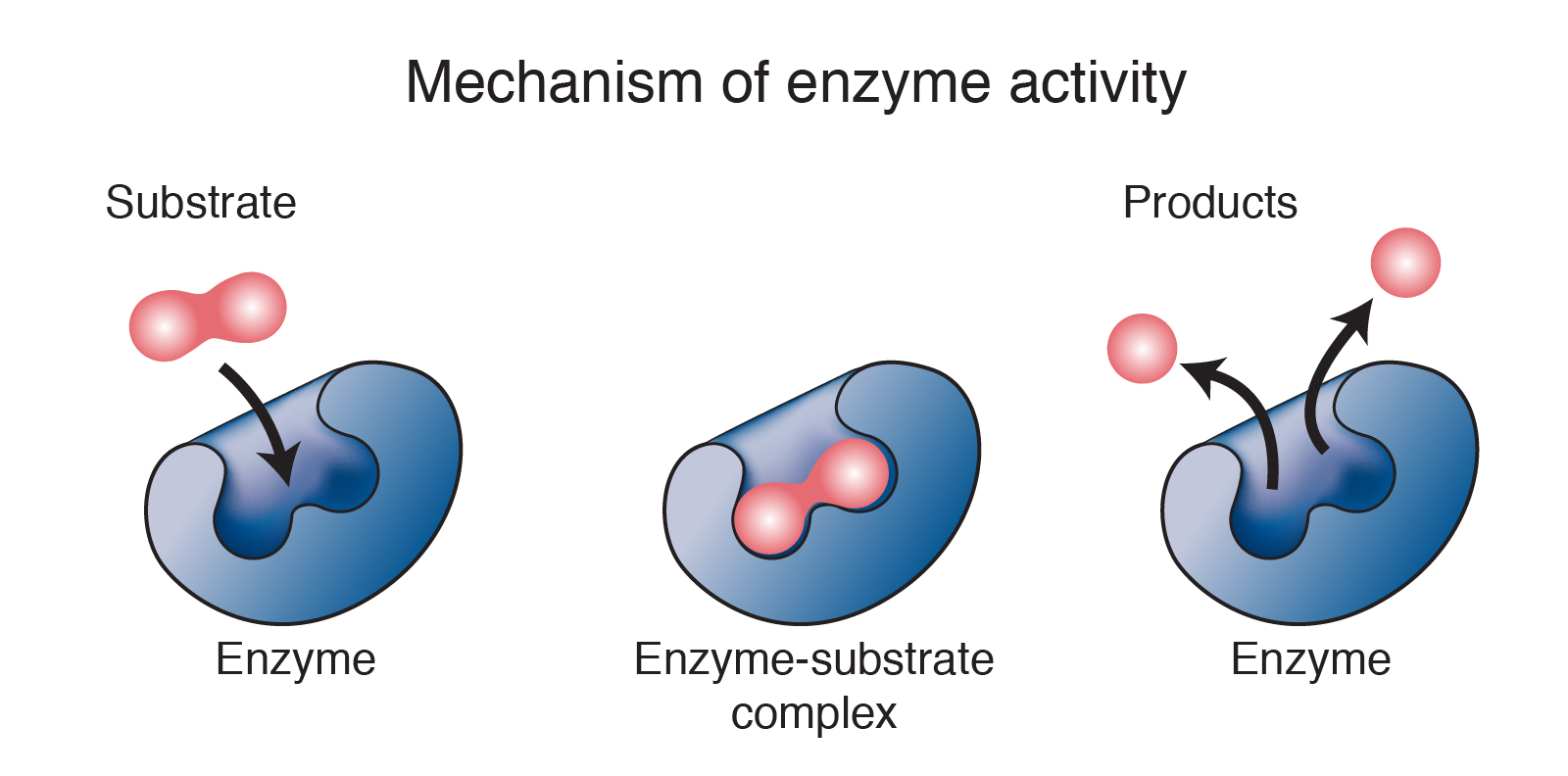
At its core, a chemical reaction involves:
- The breaking of existing chemical bonds.
- The formation of new bonds between different atoms.
Chemical reactions can be categorized into several types:
- Exothermic reactions: These release energy into the environment.
- Endothermic reactions: These require an input of energy to proceed.
- Synthesis reactions: When two or more substances combine to form a new compound.
- Decomposition reactions: A single compound breaks down into two or more elements or new compounds.
- Single displacement reactions: One element takes the place of another element in a compound.
- Double displacement reactions: Involves swapping ions between two compounds to form two new compounds.
🌟 Note: Energy changes are often significant in biological systems where reactions are tightly regulated.
The Role of Enzymes in Catalysis

Enzymes are proteins (occasionally RNA) that function as biological catalysts:
- They increase the rate of a chemical reaction without being consumed by the reaction.
- They lower the activation energy, making it easier for reactants to proceed to the transition state and form products.
- Enzymes are highly specific, often acting only on one particular substrate or a small group of related substrates.
- The specificity arises from the enzyme’s active site, where the substrate binds to form the enzyme-substrate complex.
Enzyme Activity and Properties

Enzyme efficiency is influenced by:
- Substrate concentration: Up to a point, increasing substrate concentration leads to a proportional increase in reaction rate.
- Temperature: Enzymes have an optimal temperature where activity peaks; beyond this, denaturation occurs.
- pH: Each enzyme works best at a specific pH. Deviations from this pH range can reduce enzyme activity.
- Inhibitors: Can either block the active site (competitive inhibition) or bind elsewhere, changing the enzyme’s shape (non-competitive inhibition).
⚠️ Note: Enzymes can be affected by environmental factors; maintaining optimal conditions is key for their function.
Worksheet Analysis: 2.4 Chemical Reactions and Enzymes

Here, we’ll review some common questions found in the “2.4 Chemical Reactions and Enzymes” worksheet to understand these concepts better:
Question 1: What are the characteristics of enzymes?

Answer: Enzymes have several defining characteristics:
- They are biological catalysts that speed up reactions.
- They are not consumed in the reactions they facilitate.
- Enzymes possess an active site where substrates bind.
- They lower the activation energy of reactions.
- Enzymes can be inhibited or denatured by changes in environment.
- They are generally proteins, though some are RNA (ribozymes).
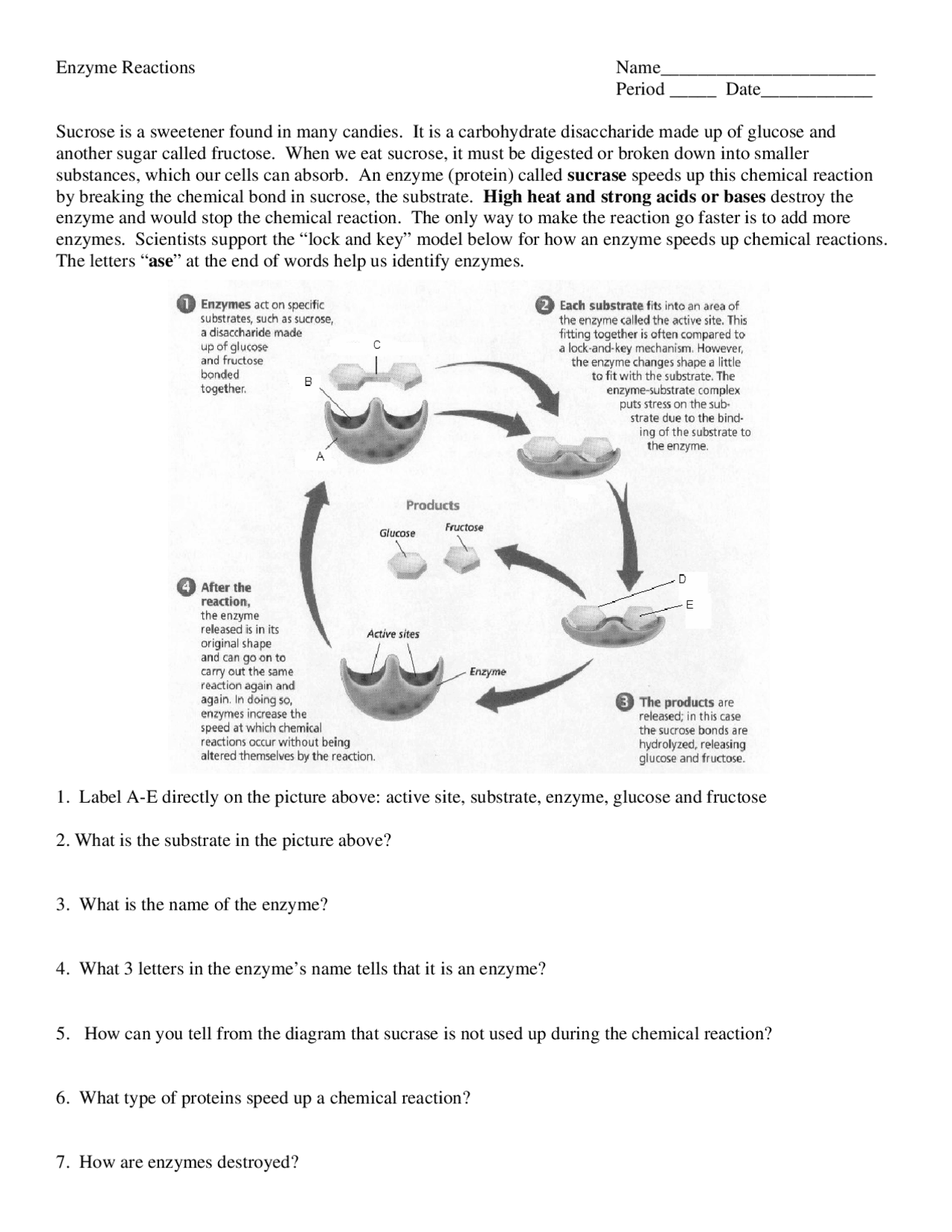
Question 2: Explain how enzymes lower activation energy.

Answer: Enzymes reduce the activation energy required to initiate a chemical reaction in the following ways:
- Orientation: By correctly aligning the substrates.
- Distortion: Stretching or otherwise altering the substrate’s shape to facilitate bond breaking.
- Transition State Stabilization: Stabilizing the transition state to reduce the energy barrier.
- Proximity Effect: Bringing reactants closer together, increasing the likelihood of collision.
🔬 Note: Understanding the mechanism of enzyme catalysis helps in predicting how enzymes might work in various conditions.
Question 3: How does pH affect enzyme activity?

Answer: Enzymes have an optimal pH at which they function most efficiently:
- Extreme pH changes: Can cause denaturation by disrupting the ionic bonds and altering the enzyme’s shape.
- Slight pH changes: Can decrease activity by affecting the substrate’s ability to fit into the active site.
- Some enzymes have a broader pH range, but many are extremely sensitive to pH variations.
FAQ:
What is the difference between a catalyst and an enzyme?

+
A catalyst increases the rate of a chemical reaction without being changed in the process. Enzymes are specific biological catalysts, made of proteins (or RNA), which facilitate chemical reactions under physiological conditions, often requiring a specific pH or temperature.
How do inhibitors work in enzyme catalysis?

+
Inhibitors reduce enzyme activity by either binding to the active site (competitive inhibition) or by binding to another site, altering the enzyme’s shape or function (non-competitive inhibition).
Can enzymes work at any temperature or pH?
+
No, enzymes are highly sensitive to temperature and pH. Each has an optimal range where it functions best. Outside of this range, the enzyme’s structure can be altered or denatured, reducing its catalytic efficiency.
Why are enzymes important in our digestive system?

+
Enzymes in the digestive system break down large food molecules into smaller, absorbable units. For example, amylase breaks down starch into sugars, protease digests proteins into amino acids, and lipase aids in the digestion of fats.
The exploration of chemical reactions and enzymes unveils the beautiful complexity of biological systems. Enzymes orchestrate a symphony of reactions within our cells, allowing life to thrive under conditions that would otherwise be inhospitable. The insights gained from studying these processes not only help us appreciate the intricate workings of life but also open doors to medical advancements, biotechnology applications, and environmental solutions. As we delve deeper into the realm of biochemistry, the answers revealed through worksheets like “2.4 Chemical Reactions and Enzymes” provide a structured path towards understanding these fundamental mechanisms.